Homeopathic Promoters to Note FTC is Revising its Guide on Endorsements and Testimonials
By Mark Land, AAHP President Of the thousands of homeopathic products in the marketplace, few are ever seen advertised on television – historically, the mass medium to blanket the large…
Your NDC Numbers are About to Change
By Eric Foxman, AAHP Secretary FDA has published a proposal that will change the required National Drug Code (NDC) format from 10 to 12 digits. Once finalized, all NDC holders…
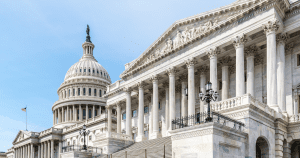
House and Senate Advance FDA User Fee Reauthorization Bills
By Pete Evich In the month of June, both the House and Senate advanced separate pieces of legislation that reauthorize FDA’s prescription drug, medical device, biosimilars, and generic drug user…
The Status of Homeopathic Medicinal Products in the European Union
By European Coalition on Homeopathic & Anthroposophic Medicinal Products (ECHAMP) Homeopathic medicinal products have a strong foundation in the EU law. Specific EU legislation for homeopathic medicinal products has…
FDA Seeks Accelerated Drug Approval, DSHEA, Cosmetics Legislative Reforms
By Pete Evich, AAHP Government Relations As part of the President’s FY 2023 Budget proposal, FDA has called upon Congress to take up legislation that would give the agency additional…
The Regulation of Homeopathic Medicines in 2022
By Mark Land, AAHP President The content in our June 2022 newsletter on regulation turned out quite different than I imagined when we discussed it at an editorial planning…
Regulation of Homeopathic Medicines in Canada
Submitted by David Skinner, Canadian Homeopathic Pharmaceutical Association Homeopathic medicines (HMs) are regulated in Canada by Health Canada, the federal government department responsible for ensuring the safety, efficacy, and…
Regulatory Status of Homeopathy in the EU & UK: What’s Happened Since Brexit?
By Steve Mann, Director of External Regulatory Affairs, Nelsons To say the world has changed over the last couple of years might just be the understatement of the century…
A Global Perspective on Effectiveness Standards for Homeopathic Medicinal Products: Is there a “Third Way”?
By Robbert van Haselen, Chair, HPCUS Clinical Documentation Committee; Director, World Integrated Medicine Forum We currently live in an increasingly polarized world, and this is also reflected in the…
Be ’Recall Ready’: FDA Wants to Help You Fulfill Your Regulatory Requirements
By Eric Foxman, AAHP Secretary Does your company market or distribute an FDA-regulated product? If so, then FDA has a document for you. FDA wants all manufacturers and downstream distribution…
AAHP monitors and reports on current federal regulations, legislation and government policies that affect the homeopathic community. AAHP is often the first to learn of regulatory changes that have both direct and indirect effects on our industry enabling members to stay ahead of the curve in a rapidly changing healthcare environment.
23 Comments
Leave a Comment
You must be logged in to post a comment.









2equitable
pittsburgh gay chat https://bjsgaychatroom.info/
gay dad dating game https://gaypridee.com/
gay chat nyc https://gaytgpost.com/
gay dirty chat https://gay-buddies.com/
list of gay dating sites https://speedgaydate.com/
best time to play slots https://2-free-slots.com/
5.oo slots machine casino https://freeonlneslotmachine.com/
bio dr. jorgen slots https://candylandslotmachine.com/
free slots games for fun https://pennyslotmachines.org/
free igt slots https://slotmachinesworld.com/
free triple diamond slots https://slotmachinesforum.net/
free vegas penny slots https://slot-machine-sale.com/
penny slots https://beat-slot-machines.com/
quick hits free slots https://411slotmachine.com/
hollywood slots bangor https://www-slotmachines.com/
free giants gold slots https://slotmachinegameinfo.com/
help-seeking dissertation https://buydissertationhelp.com/
online dissertation help to write https://dissertationwriting-service.com/
best dissertation writing services uk https://help-with-dissertations.com/
help me with my dissertation https://mydissertationwritinghelp.com/
example of a dissertation https://dissertations-writing.org/
leeds university dissertation help https://helpon-doctoral-dissertations.net/