United States Food and Drug Administration Office of Pharmaceutical Quality
 By Mark Land, AAHP President
By Mark Land, AAHP President
May 1, 2017
The Office of Pharmaceutical Quality (OPQ), created in Jan. 2015, enhances the U.S. Food and Drug Administration Center for Drug Evaluation and Research’s (FDA CDER) Quality Initiative. It creates a drug quality program as robust as the programs the agency already has in place for drug safety and efficacy. OPQ’s motto is “One Quality Voice.”
CDER’s Quality Initiative began more than 15 years ago when the agency first introduced the 21st Century Initiative to modernize FDA’s regulation of the pharmaceutical quality of drugs. The initiative included issues related to product quality, with current good manufacturing practices as important tools for improving overall product quality.
In 2004, FDA published its final report on pharmaceutical quality for the 21st century, laying out a vision to modernize the regulation of pharmaceutical manufacturing and enhance product quality. Director of FDA CDER Janet Woodcock said of the Quality Initiative, “the realization of this vision will result in a maximally efficient, agile, flexible manufacturing sector that reliably produces high-quality drug products without extensive regulatory oversight.”
The Food and Drug Administration Safety and Innovation Act of 2012 (FDASIA) further enhanced CDER’s Quality Initiative by directing FDA to, among other things, improve its risk-based surveillance inspection schedule of manufacturing facilities. Some outgrowths of FDA’s Quality Initiative ultimately codified in FDASIA and prior legislation include: risk-based approach to quality, the quality systems approach to establishment inspections, the quality metrics program and the foreign establishment inspection program.
OPQ’s mission is to address gaps in drug quality and ensure that quality medicines are available for the American public, with a vision of being a global benchmark for the regulation of pharmaceutical quality. OPQ strives to improve drug product quality by encouraging the modernization of manufacturing technologies and using proactive, collaborative approaches to identify and mitigate quality issues before they lead to drug shortages or recalls.
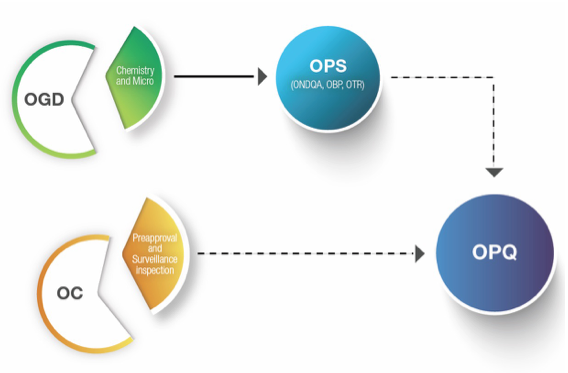
OPQ emerged from a reorganization of quality activities from the Office of Generic Drugs (OGD), Office of New Drugs (OND) and the Office of Compliance (OC). The organizational goal was to place evaluation and decision-making on quality under one office. Michael Kopcha, Ph.D., R.Ph., is the director of OPQ.
The Office of Program and Regulatory Operations (OPRO) is accountable for leading and coordinating regulatory review processes, facilitating a quality management system. The office also responsible for developing and maintaining a learning and professional development program in collaboration with review offices within OPQ.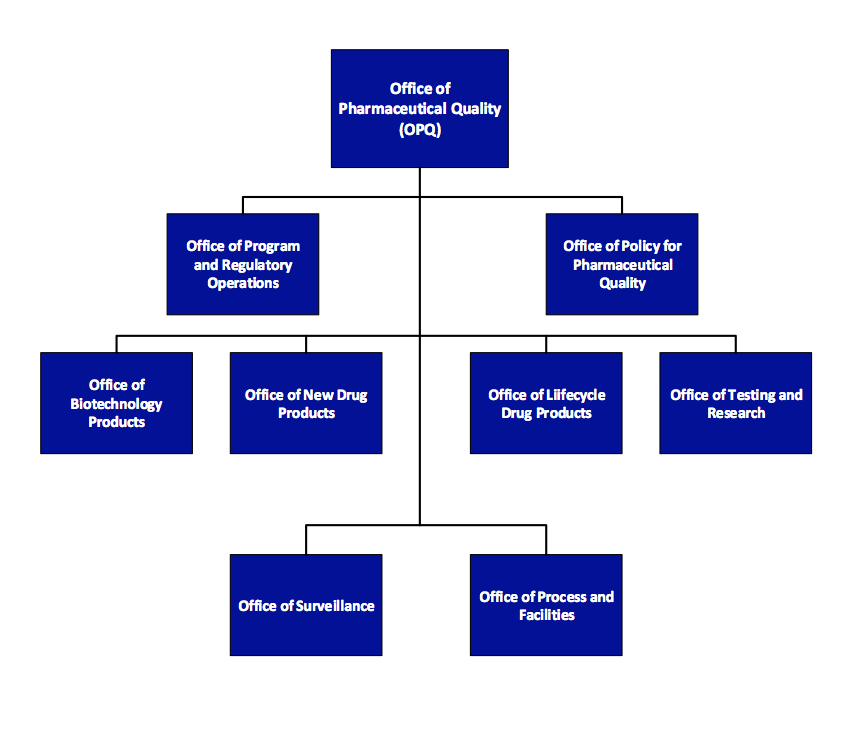 The Office of Policy for Pharmaceutical Quality (OPPQ) develops, implements and updates science- and risk-based policies, standards and guidance documents, including chemistry, manufacturing and controls (CMC) review policy and CGMP/ inspection policy and standards. OPPQ is responsible for communication between Agency and external stakeholders (e.g., Government Accountability Office, Congress, public, industry, pharmacopeias, and other government agencies).
The Office of Policy for Pharmaceutical Quality (OPPQ) develops, implements and updates science- and risk-based policies, standards and guidance documents, including chemistry, manufacturing and controls (CMC) review policy and CGMP/ inspection policy and standards. OPPQ is responsible for communication between Agency and external stakeholders (e.g., Government Accountability Office, Congress, public, industry, pharmacopeias, and other government agencies).
Within OPQ, quality review of drug substance, drug product, and biopharmaceutics will mainly reside in the Office of Biotechnology Products (OBP), Office of New Drug Products (ONDP), and Office of Lifecycle Products (OLDP). Reviewers in these three offices will assess drug substance, drug product formulation and specification, and data from exhibit or clinical batches.
The Office of Process and Facilities (OPF) evaluates pharmaceutical manufacturing process, design and controls for their ability to be successfully implemented at a commercial scale. Process and facility reviewers may participate in pre-approval inspections (PAIs) to ensure the proposed control strategy is appropriately implemented.
Quality surveillance enhances FDA’s ability to monitor quality across facilities, providing impetus for both FDA and industry to respond quickly to process trends before serious quality problems can occur. OPQ has identified a set of quality metrics for use in surveillance so the FDA can better monitor and prioritize facilities for risk-based surveillance inspection.
Since its creation, the OPQ has established or taken charge of 65 quality guidance documents including revision of the guidance for botanical drugs. It has also developed the quality metrics program and become a driver of efficiency at FDA in its review of drug product applications.
Rik Lostritto, Ph.D., of OPQ’s Office of Policy for Pharmaceutical Quality, is the lead liaison for the pharmaceutical industry. He has made several presentations to the homeopathic industry, often reporting on validation and process control matters. OPQ is the organizational unit within FDA charged with elevating industry and FDA’s understanding of risk management in pharmaceutical manufacturing. AAHP members may not directly interface with OPQ, but their work informs establishment inspection plans and protocols. Maintaining surveillance on the work of OPQ and understanding their objectives should be part of your regulatory intelligence program.
References:
- United States Food and Drug Administration CDER’s Quality Initiative. Available at https://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDER/ucm417014.htm.
- United States Food and Drug Administration Pharmaceutical CGMPS for the 21st Century – A Risk Based Approach Report. Available at https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/Manufacturing/QuestionsandAnswersonCurrentGoodManufacturingPracticescGMPforDrugs/UCM176374.pdf.
- United States Food and Drug Administration Pharmaceutical Quality Oversight. Available at https://www.fda.gov/downloads/aboutfda/centersoffices/officeofmedicalproductsandtobacco/cder/ucm442666.pdf.
- Food and Drug Administration Safety and Innovation Act Public Law 112–114 July 9, 2012.
- FDA/CDER SBIA Chronicles April 4, 2016. Available at https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/SmallBusinessAssistance/UCM494312.pdf.
