Poison Control Center Statistics on Homeopathy
What’s Your Company’s Safety Statistics?
When FDA announced its public hearing on homeopathy, the agency cited 10,311 reported poison exposure cases related to “homeopathic agents” in the 2012 American Association of Poison Control Centers Annual Report. This article will introduce a new program by the National Capital Poison Center (NCPC) to ensure that consumers receive accurate information and advice for unintentional and worrisome exposures to your company’s products. It will also look at AAHP’s response to statistics from the AAPCC 2012 report, and an eight-year trend in the numbers.
webPOISONCONTROL: A New Tool
Each year, millions of people reach out to the experts at poison control for help when they are exposed to common household substances, including over-the-counter drugs. In keeping with the times, NCPC — a 501(c)(3) charitable organization — developed online guidance for those who prefer not to call or can’t call. webPOISONCONTROL provides instant, free triage recommendations and automated follow up for acute, unintentional exposures to a vast array of products. The new tool, which is also in the form of an app, is used by 24 of the 55 poison control centers across the country.
How to Increase Your Products’ and Homeopathy’s Safety Profile Accuracy
Homeopathic manufacturers have an opportunity to provide proprietary formulas to NCPC, which will remain confidential, so that automated medical recommendations are as appropriate as possible. Providing this information could make the difference between consumers being advised to stay at home and watch for symptoms or to go to an emergency room. Up-to-date product pictures, labels, names, and UPCs can also help both consumers and experts at poison control centers confirm the involvement of your product in an exposure. Contributing these materials through a partnership with NCPC may also increase homeopathy’s safety profile overall among government agencies and news outlets.
Benefits to an NCPC Partnership:
- Consumer Safety Information Accessibility: Ensures your consumers can readily get the expert help they need for unintentional and worrisome exposures to your products.
- Reputation Management: Helps refute inaccurate safety information about your products with accurate information from a trusted, unbiased resource.
- Product Safety Liability Mitigation: Demonstrates that your company has taken extra initiative to ensure its consumers have access to safety information on its products.
- Social Responsibility and Product Stewardship: Shows your commitment to corporate social responsibility and product stewardship.
- Insight: Provides insight into unintentional exposures that are not captured by calls to poison control. Data from a different perspective can further inform your company’s public education and safety efforts.
Private individual exposure surveillance dashboards allow NCPC partners to review cases by:
- Age and gender
- Geography
- Trends in frequency
- Type of advice provided by the app
- Final outcome
- Follow-up by ages in some cases
- Route of exposure and symptoms may also be available
What’s Your Company’s Safety Statistics?
webPOISONCONTROL case data are kept separate from the National Poison Data System (NPDS), which contains data on poison control telephone calls only. Homeopathic manufacturers who are curious about the cases that involve their company’s products and the data that drive the recommendations provided by the app can contact Krista Osterthaler, MPH (LinkedIn), NCPC Director of National Strategic Alliances, at Osterthaler@POISON.org.
AAHP-NCPC Feb. 9 Event
Ms. Osterthaler will host a complimentary one-hour presentation on Feb. 9, 2021 on how NCPC determines the potential toxicity of homeopathic products and advises consumers. She will also demonstrate a private exposure surveillance dashboard that allows NCPC partners to receive statistics about their products. This complimentary informational event is open to all homeopathic manufacturers (not limited to AAHP members). Register for the event through NCPC Go-to-Webinar here. (Register early to secure one of the limited spots.)
Reputation of Homeopathic Products
The March 27, 2015 Federal Register announced that FDA would hold a public hearing to evaluate the agency’s then 25-year regulatory framework of homeopathic products. This set in motion six years of FDA working to revise its guidance, resulting in sensationalized news headlines with every procedural announcement.
Statistics for Homeopathic Agents Compiled from the 2012–2019 Annual Reports of the American Association of Poison Control Centers’ National Poison Data System (NPDS)
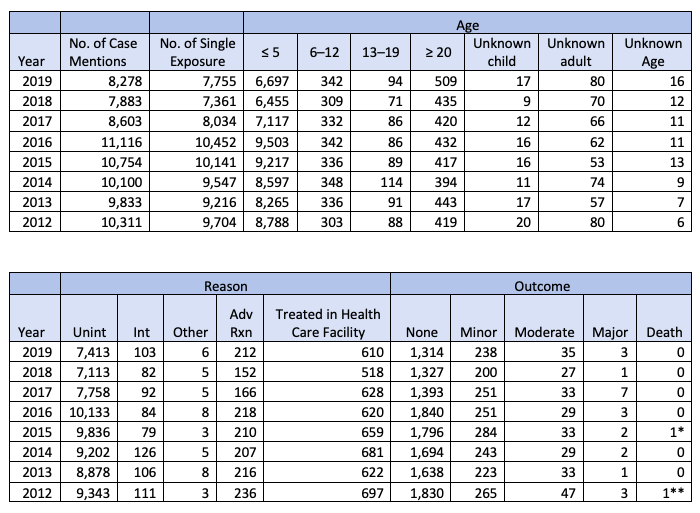
Table 22B: Demographic profile of SINGLE SUBSTANCE pharmaceuticals exposure case by generic category.
Among other background, the Federal Register announcement cited:
“The 2012 American Association of Poison Control Center Annual Report indicated that there were 10,311 reported poison exposure cases related to ‘‘Homeopathic Agents,’’ with 8,788 of those reported cases attributed to children 5 years of age and younger (Ref. 3). Of the 10,311 reported cases, 697 required treatment in a health care facility (Id.).”
An analysis of this data was presented at the FDA hearing (by Edward P. Krenzelok, Pharm.D., National Poison Data System Summary of Reported Homeopathic Exposures, 2005–2014). The analysis showed that the number of exposures to homeopathic medicines in any given year is less than one percent of all pharmaceutical reports to AAPCC, which is proportionally below the rate of the market share for homeopathic medicines.
This analysis also shows that most exposures were managed outside of a healthcare facility, with only one percent of all exposures resulting in admission. Related clinical effects were reported in five percent of all exposures, with vomiting the most common clinical effect reported (one percent of all exposures). Of exposures followed to a known medical outcome, 86 percent resulted in no effect or an effect deemed unrelated to the homeopathic product.
News outlets reported the statistics but not the analysis.
webPOISONCONTROL uses its own custom-built substance and product database but uses the same coding classification as NPDS to facilitate dataset comparison. As of Dec. 10, 2020, webPOISONCONTROL has managed more than 503,000 cases since 2014, 202,114 (40 percent) of which implicated a pharmaceutical product. A product classified as a “Dietary supplement / herbal / homeopathic” product was implicated in 25,183 cases, 79 percent of which received advice for home-management because potential toxicity was deemed minimal. Just 0.06 percent of these cases were advised to seek evaluation in an emergency department.
An industrywide partnership with the National Capital Poison Center for webPOISONCONTROL would reduce worry and costs of unnecessary emergency room visits and help homeopathic product safety statistics. AAHP encourages all homeopathic manufacturers to get involved with webPOISONCONTROL.
* Details not found in report. Despite one death reported on Table 22 (above), the category “Dietary Supplements/Herbals/Homeopathic” does not appear within the 2015 “Table 21. Listing of fatal nonpharmaceutical and pharmaceutical exposures”.
** There are two cases listed on the 2012 “Table 21. Listing of fatal nonpharmaceutical and pharmaceutical exposures” under “Dietary Supplements/Herbals/Homeopathic”. Case [1812pa] involved Yohimbe. Case 1813h involved “supplement, botanical” and cocaine. One of these substances may have been miscategorized as being a homeopathic agent.
