Safe Drugs, Happy Consumers: Sterility Assurance Programs
By Kristina Skowronek, Director of Quality and Regulatory Compliance, Boiron USA
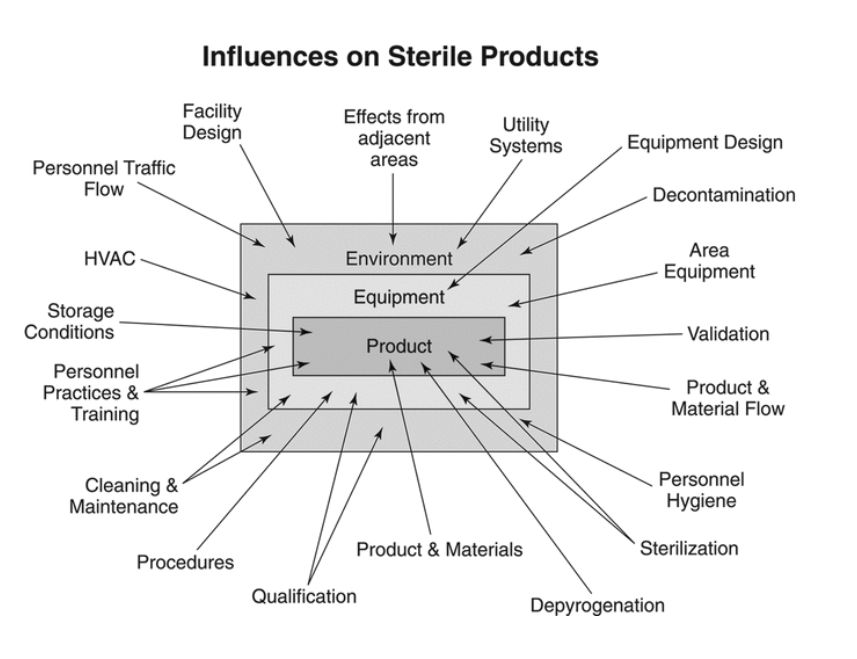
If we were to manufacture a drug product that is purported to be sterile but cannot be terminally sterilized without ingredient or package degradation, a sterility assurance program would be absolutely critical to its strength, identity, safety, purity, and quality. A strong sterility assurance program has many layers. Each stage in manufacturing and packaging has to be carefully managed via validated processes, strong adherence to well-developed standard operating procedures, and both in-process and finished-product testing. Risk analysis can help a manufacturer identify steps during which there is a risk of contamination throughout a manufacturing process so that controls may be developed to mitigate those risks.
There are clear standards set forth in the United States for sterility assurance. The U.S. Food and Drug Administration (FDA) has a published guidance document for industry: Sterile Products Produced by Aseptic Processing — Current Good Manufacturing Process (September 2004). The process areas addressed within this guidance include the following: Buildings and Facilities, Personnel Training, Qualification, Components and Container Closures, Endotoxin Control, Time Limitations, Validation of Aseptic Processing and Sterilization, Laboratory Controls, and Sterility Testing. The impact of these areas on the process and on the product should be examined closely. The U.S. Pharmacopoeia also examines all areas of impact on a sterile product or process in USP General Chapters, General Information, <1211> Sterility Assurance.
The facility in which an aseptic manufacturing process is to take place must be fit for its intended purpose. The design and materials, air filtration, janitorial program, and equipment must all be installed and maintained so as to prevent microbial contamination. FDA also addresses these elements in various sections within 21 CFR 211. Rooms in which aseptic processing will occur must also meet the specifications provided in the USP General Chapters <1116> Microbiological Control and Monitoring of Aseptic Processing Environments. Clean room classifications, the specifications for these classifications, and the microbiological testing methods to control the rooms are outlined. Air and surfaces are routinely monitored within rooms to assure they continuously meet specifications. Janitorial practices must be involved in a cleaning validation program and microbiological controls need to be routinely done to assure routine cleaning adheres to specifications.
To maintain a clean room environment, sterility must be assured for cleaning solutions, tools and equipment, raw materials, container closures, and any other materials that will be introduced into the area such as compressed air or water. If sterile air is used, proper filtration is to be in place with routine qualification and control of filters and the air itself. Water for Injection is typically used for solutions involved in cleaning activity, disinfectants, and in the product itself. Control of the water system is also crucial to prevent contaminants from entering the environment or the product. USP <1231> Water for Pharmaceutical Purposes provides an overview of types of water used, and individual monographs address specifications and requirements for water systems based upon their intended purpose.
Personnel who will work in clean rooms during aseptic processing must have the appropriate training or education and qualification. Procedures should be in place to clearly define the processes by which personnel may be qualified to wear gowning materials, how to utilize aseptic technique, how to clean or sterilize materials, how to conduct themselves in a clean room, and how to identify and respond to non-conformances. Re-training and qualification of personnel should be done on a routine basis to keep them sensitized to the importance of aseptic technique. This may be done via a combination of procedural training, microbiological monitoring of gowning, and participation in media fill trials that can be used to validate personnel technique as well as the aseptic process.
Validation of an aseptic process is done by conducting media fills with environmental microbiological monitoring. An appropriate microbiological media will be treated as if it were product and it will be put through an entire manufacturing process simulation. This study will show contamination control effectiveness throughout the manufacturing process. The study shall include all steps from set-up through manipulations during the course of a normal production run to completion of the batch. All finished units from a trial are incubated and examined for microbial contamination. Interpretation of results includes for batch sizes of 10,000 or more units, one positive unit should generate a full investigation and two positive units require investigation and re-validation. Initial qualification includes a minimum of three successful consecutive runs followed by periodic re-qualification to ensure consistency and control over the process. All aseptic area personnel, including maintenance personnel, should be qualified at least annually by participation in a media fill. Any changes to the process must be governed by a change control and are to be re-validated via a set of initial media fills.
Laboratory controls are critical to sterility assurance. A sterile product is to undergo analysis for microbial endotoxins and sterility testing to assure the absence of contamination. USP General Tests and Assays provides guidelines, specifications, and test methods to utilize such as: USP <71> Sterility Tests and USP <85> Bacterial Endotoxins Test. For these tests as well as environmental microbiological monitoring, proper media shall be used that has undergone growth promotion testing to ensure it will detect the possible microbes. Unusual results or out-of-specification results must be investigated to identify the microbes detected, determine their source, and perform an assessment of impact on product quality and safety.
This high-level discussion of sterility assurance merely scratches the surface of an effective program. Proper assurance may be obtained if risks at all levels of the operation are identified and addressed by procedures and processes that are clear, based on accepted standards, and strictly followed. Consumers’ health depends upon it.
Kristina is the Director of Quality and Regulatory Compliance for Boiron USA where she formerly served as QA Manager and has managed quality control, quality assurance, and regulatory compliance activities throughout her 13 years for this subsidiary with global headquarters in France. She previously spent four years with Wyeth (now Pfizer) as a Microbiologist and Quality Control Supervisor managing the microbiological monitoring program for ISO class 8, 7 and 5 facilities as well as media fills for a parenteral penicillin operation. Kristina holds a B.S. in Biology, a Master’s in Education, and expects to complete an M.S. in Regulatory Affairs and Quality Assurance from Temple University in August 2019.
