Regulatory Policy Analysis
A Tool for Evaluating the Regulatory Framework for Homeopathic Drug Products
By Mark Land, AAHP President
Today the combination of drug regulations, approval options, cost controls and societal health care goals yield outcomes that are no longer obvious or predictable. So complex is the process that the field of regulatory science emerged to address the range of development options, analytical tools, surrogate end points and approval pathways. Regulatory science is itself a complex of disciplines including regulatory science, registration science and regulatory policy analysis. Of the three areas, regulatory policy analysis may have the greatest application in today’s regulatory environment for homeopathic drug products.
Regulatory Science
According to the U.S. Food and Drug Administration (FDA), “Regulatory Science is the science of developing new tools, standards, and approaches to assess the safety, efficacy, quality, and performance of all FDA-regulated products.”1 In practice this includes translational science, biomarkers and accelerated approval pathways to name some of the most important. The goal is to accelerate approval by reducing uncertainty in decision-making.
Registration Science
The International Center for Regulatory Science at the University of Southern California (USC) uses this definition: “Regulatory Science relates the regulatory and legal requirements of biomedical product development to the scientific research needed to ensure the safety and efficacy of those products.”2 This program develops expertise in the requirements of drug regulatory pathways including application requirements, scientific expectations of regulators and global regulatory registration strategy. This discipline aims to ensure marketing approval.
Regulatory Policy Analysis
While Bert Leufkens of the Dutch Medicines Evaluation Board and Utrecht University does not specifically use the term “regulatory policy analysis,” it seems inherent in his thoughts on the role of regulatory science: “…regulatory science should evaluate and study regulatory systems in terms of their ability to ensure patient safety, enhance public health, and stimulate innovation.”3 Regulatory policy analysis seeks to compare the direct and unintended outcomes of regulatory decisions to the original policy goals.
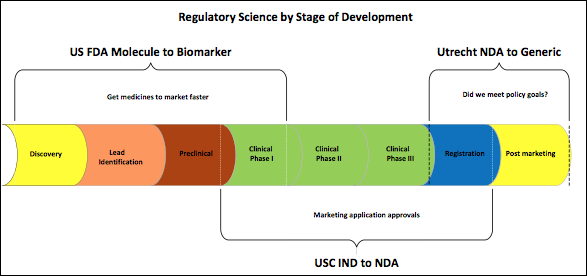
Taken together we see the progression of these disciplines beginning with discovery through the evaluation of outcomes at the policy level (see Figure 1). The FDA considers regulatory science to be an adjunct to translational science. How can we use what we know about physiology to get the molecule from patent to patient as quickly as possible with the least uncertainty? At USC they train students as intermediaries between scientists in the health care company and regulatory authorities. At Utrecht University regulatory science is positioned post-marketing with the aim of evaluating outcomes of policy decisions in relation to the original goals of the population served.
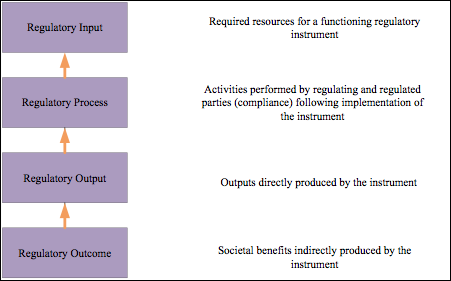
Regulatory and registration science are important disciplines. Regulatory policy analysis however, may have the greatest application in questions for homeopathic drug product regulation today. The tools of policy analysis are used to identify and evaluate new areas of thought that may be of help to policy makers. Bouvy’s4 regulatory policy analysis flowchart (Figure 2) has been reversed to illustrate the potential of thinking from the perspective of societal benefits. Working from the bottom up focuses on potential outcomes as the foundation for development of regulatory tools.
The FDA opened a docket to examine the regulatory framework for homeopathic drug products after a quarter of a century.5 In its introduction to this topic, the FDA asked eight questions. In summary, the agency wanted to know: what are suitable indications for non-prescription homeopathic drug products; are we satisfied with the labels of these products; and how, if at all, should their regulatory framework be changed?
The policy perspective implied through these questions is: how does the FDA ensure that only safe, high-quality, well-labeled homeopathic drug products reach the market?
The American Association of Homeopathic Pharmacists (AAHP) in comments to FDA6 outlined its recommendation for enforcement policy as follows:
- Require that homeopathic active ingredients be referenced within the Homeopathic Pharmacopoeia of the United States;
- Prohibit combinations of homeopathic active ingredients with other ingredients;
- Identify names of active ingredients in Latin;
- Identify the product as homeopathic on the principal display panel; and
- Require that labels bear a “Not Reviewed by FDA” disclaimer.
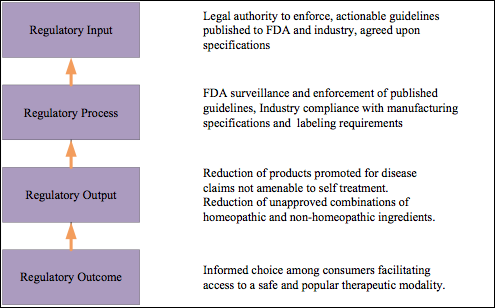
Analysis of the AAHP proposal using Bouvry’s approach reveals a potential gap in the FDA’s legal authority to enforce compendial and label requirements (Figure 3).
Regulatory policy analysis can be an effective approach when evaluating regulations in place or under development. Predicting societal level outcomes of policy decisions is difficult. Using a systematic approach to evaluate and challenge policy goals can be a useful tool when navigating regulatory decisions.
References
- United States Food and Drug Administration Advancing Regulatory Science. Available at: http://www.fda.gov/scienceresearch/specialtopics/regulatoryscience/default.htm.
- The International Center for Regulatory Science at the University of Southern California. Available at: https://regulatory.usc.edu/international-center/.
- Huub Schellekens, Ellen Moors, H.G. Leufkens Regulatory Systems Science 2011 April 8; 332 (6026): 174 -5.
- Bouvry et al 2014, Escher TI-Pharma. Available at: http://www.tipharma.com/pharmaceutical-research-projects/regulatory-innovation/escher.html.
- United States Food and Drug Administration Homeopathic Product Regulation: Evaluating the Food and Drug Administration’s Regulatory Framework After a Quarter-Century; Public Hearing. Available at: https://www.federalregister.gov/documents/2015/03/27/2015-07018/homeopathic-product-regulation-evaluating-the-food-and-drug-administrations-regulatory-framework.
- Comments of the American Association of Homeopathic Pharmacists. Available at: https://www.regulations.gov/document?D=FDA-2015-N-0540-9241.